Muscle–Organ Crosstalk: The Emerging Roles of Myokines
Physical activity decreases the risk of a network of diseases, and exercise may be prescribed as medicine for lifestyle-related disorders such as type 2 diabetes, dementia, cardiovascular diseases, and cancer. During the past couple of decades, it has been apparent that skeletal muscle works as an endocrine organ, which can produce and secrete hundreds of myokines that exert their effects in either autocrine, paracrine, or endocrine manners.
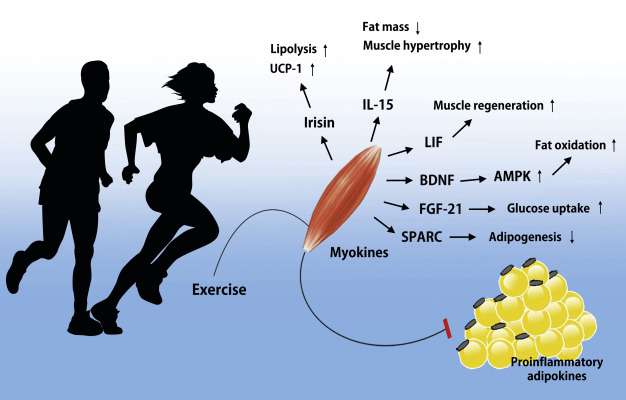
Recent advances show that skeletal muscle produces myokines in response to exercise, which allow for crosstalk between the muscle and other organs, including brain, adipose tissue, bone, liver, gut, pancreas, vascular bed, and skin, as well as communication within the muscle itself. Although only few myokines have been allocated to a specific function in humans, it has been identified that the biological roles of myokines include effects on, for example, cognition, lipid and glucose metabolism, browning of white fat, bone formation, endothelial cell function, hypertrophy, skin structure, and tumor growth. This suggests that myokines may be useful biomarkers for monitoring exercise prescription for people with, for example, cancer, diabetes, or neurodegenerative diseases.
The role of myokines has previously been reviewed, identifying more than 650 myokines . Some myokines are responsible for mediating energy supply in relation to acute bouts of exercise. Myokines are also involved in muscle proliferation, differentiation, and regeneration independent of exercise. During exercise, myokines signal within the muscle and mediate muscle–organ crosstalk to the brain, adipose tissue, bone, liver, gut, pancreas, vascular bed, and skin. In addition, myokines with anticancer effects have been recognized.
- Myokines are defined as cytokines and other peptides that are produced, expressed and released by muscle fibers and exert either autocrine, paracrine, or endocrine effects
- Myokines mediate communication between muscle and other organs, including brain, adipose tissue, bone, liver, gut, pancreas, vascular bed, and skin, as well as within the muscle itself.
- Myokines exert their effects on, for example, cognition, lipid and glucose metabolism, browning of white fat, bone formation, endothelial cell function, hypertrophy, skin structure, and tumor growth.
- The myokine IL-6 mediates the exercise-associated anti-inflammatory effects both acutely with each bout of exercise and as a consequence of training adaptation, including reduction in abdominal adiposity.
- The identification of new myokines and their specific roles may lead to novel therapeutic targets.
- Myokines can be useful biomarkers for monitoring the type and amount of exercise that are required for the prescription of exercise for people with, for example, cancer, diabetes, or neurodegenerative diseases.
Muscle–Brain Crosstalk
Evidence is accumulating that physical exercise has positive health effects on cognitive function and brain health. Physical activity and exercise training decrease the risk of dementia and appear to play a role in the treatment of this disease. In general, it is found that physical activity decreases the rate of cognitive decline in healthy people and in people with neurodegenerative disorders across the life span. Moreover, physical exercise has a positive impact on stress, anxiety, and depression. Other studies have shown that an active lifestyle is associated with learning and memory, executive functions, language and reaction time, academic achievement in children, and intelligence in adolescents. Physical activity has also beneficial effects on appetite, sleep, and mood.
Exercise has been shown to influence the hippocampus more than any other part of the brain. Studies in rodents and humans have shown that exercise increases hippocampus volume and the blood flow to this part of the brain. In particular, exercise has been shown to influence neurogenesis in the dentate gyrus and to increase synapse plasticity.
The finding that muscle contraction is sensed by the brain suggests that peripheral factors induced by exercise may be involved in direct crosstalk between working muscle and brain function.
Muscle–Bone Crosstalk
Muscle and bone are closely related during development growth, and muscle disuse and/or muscle atrophy result in osteoporosis. As pointed out by Guo et al, muscle mass, measured as lean body mass, can only explain up to 20% of the variety in bone mineral density and decreased mechanical loading, as seen with muscle atrophy alone, is not likely to fully explain the loss of bone mass. It is obvious that bone mass could also be regulated by muscle-derived biochemical factors such as myokines.
Muscle–β-Cell Crosstalk
It is well established that exercise can enhance insulin sensitivity, whereas it is less clear whether exercise can improve insulin secretion and whether a communication exists between insulin-resistant skeletal muscle and pancreatic β-cells.
Muscle–Skin Crosstalk
Aging is associated with numerous alterations, including changes of the skin. Tarnopolsky and colleagues demonstrated that endurance exercise improves age-associated skin changes in both mice and humans. They showed that exercise regulates muscular IL-15 expression via skeletal muscle AMPK. Elimination of muscle AMPK led to a weakening of skin structure, whereas IL-15 injections mimic some of the anti-aging effects of exercise on murine skin. The study supports the idea that exercise retards skin aging via a mechanism that involves muscle-derived IL-15
Muscle–Immune Inflammation Crosstalk
During exercise, muscle works as an immunoregulatory organ with impact on leukocyte subset trafficking and inflammation.
The anti-inflammatory effects of exercise
Physical inactivity is associated with low-grade chronic inflammation, not least when a physical inactive lifestyle is associated with obesity.
In humans, exercise training can induce anti-inflammatory effects both acutely with each bout of exercise and via long-term training adaptation including reduction in abdominal adiposity. The exercise-induced acute increase in IL‐6 stimulates an anti-inflammatory systemic environment. Thus, IL-6 promotes an increase in the production of the anti-inflammatory cytokines, IL‐1 receptor antagonist (IL‐1ra) and IL‐10. IL‐1ra inhibits IL‐1β signal transduction and IL‐10 inhibits synthesis of TNF‐α
Muscle–Cancer Crosstalk
Epidemiological studies suggest that physical activity in leisure time reduces the risk of at least 13 different cancer types. People who are physically active after a diagnosis of prostate cancer, colorectal cancer, and breast cancer have a higher survival rate than physically inactive people suffering from the same cancer types. It is obvious, that many cancers are accompanied by systemic low-grade chronic inflammation and that such inflammation may drive tumor progression. Therefore, the anti-inflammatory effects of physical training may mediate some of the protective effects of exercise on cancer development.

